Clinical trial design
The participants were divided into 2 groups:
MYQORZO was studied in a 24-week, Phase 3, randomized, double-blind, placebo-controlled clinical trial. It is the largest obstructive hypertrophic cardiomyopathy clinical trial of its kind to date.
- The majority of participants (85%) were already taking other medicines for their obstructive hypertrophic cardiomyopathy (beta blockers, calcium channel blockers, and/or disopyramide)
- The majority of participants (85%) were already taking other medicines for their obstructive hypertrophic cardiomyopathy (beta blockers, calcium channel blockers, and/or disopyramide)
Clinical trial results
IN THE CLINICAL TRIAL,
MYQORZO was shown to increase exercise capacity
The group taking MYQORZO had a significant improvement in exercise capacity, as measured by the average change in peak oxygen consumption (peak VO2), compared with the placebo group.
Peak VO2 improvement of 1 mL/kg/min has been shown to improve outcomes and make a clinically meaningful difference for people with obstructive HCM
Change in exercise capacity after 24 weeks

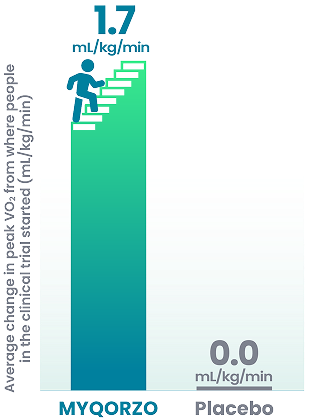
Peak VO2 improvement of 1 mL/kg/min has been shown to improve outcomes and make a clinically meaningful difference for people with obstructive HCM
Additional clinical trial results
High blood pressure (hypertension) was the only side effect reported in the clinical trial in more than 5% of patients and more commonly in patients taking MYQORZO (8%) than placebo (2)%.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about MYQORZO?
MYQORZO can cause serious side effects, including:
Heart failure, a condition where the heart cannot pump with enough force, is a serious condition that can lead to death. You must have echocardiograms (echos) before and during treatment with MYQORZO and monitor for signs and symptoms of heart failure. People who develop a serious illness such as a serious infection or who develop a new or worsening irregular heartbeat have a greater risk of heart failure during treatment with MYQORZO
Tell your healthcare provider or get medical help right away if you develop new or worsening shortness of breath, chest pain, fatigue, leg swelling, a racing sensation in your heart (palpitations), or rapid weight gain.
- The risk of heart failure is also increased when MYQORZO is taken with certain other medicines. Tell your healthcare provider about any prescribed and over-the-counter medicines you take, before and during your treatment with MYQORZO
Because of the risk of heart failure, MYQORZO is only available through a restricted distribution program called the MYQORZO Risk Evaluation and Mitigation Strategy (REMS) Program
- Your healthcare provider must be enrolled in the MYQORZO REMS Program for you to be prescribed MYQORZO
- Before you start treatment with MYQORZO, you must enroll in the MYQORZO REMS Program. Talk to your healthcare provider about how to enroll in the program. You will be given information about the program when you enroll
- Before you take MYQORZO, your healthcare provider and pharmacist will make sure you understand how to take MYQORZO safely, which will include returning for echos when advised by your healthcare provider. MYQORZO can only be dispensed by a certified pharmacy that participates in the MYQORZO REMS Program
- If you have any questions about the MYQORZO REMS Program, ask your healthcare provider, go to www.MYQORZOREMS.com, or call 1-844-285-73671-844-285-7367
Who should not take MYQORZO?
Do not take MYQORZO if you take a medicine called rifampin.
What are the possible side effects of MYQORZO?
MYQORZO can cause serious side effects, including heart failure.
What should I tell my healthcare provider before taking MYQORZO?
Before taking MYQORZO, tell your healthcare provider about all of your medical conditions, including if you:
- Are pregnant or plan to become pregnant. It is not known if MYQORZO can cause harm to your unborn baby. Tell your healthcare provider if you become pregnant during treatment or within 3 weeks after the last dose of MYQORZO. There is a pregnancy study for MYQORZO. Your healthcare provider should report your pregnancy exposure to Cytokinetics, Inc
- Are breastfeeding or plan to breastfeed. It is not known if MYQORZO passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with MYQORZO
Before and during MYQORZO treatment, tell your healthcare provider about all the prescription and over-the-counter medicines, vitamins, and herbal supplements you take. Taking MYQORZO with certain medicines may lead to increased levels of MYQORZO in your blood and increase the risk of heart failure. Do not stop or change the dose of a medicine or start a new medicine without telling your healthcare provider.
Especially tell your healthcare provider if you take fluconazole (if used for more than 3 days), voriconazole, or fluvoxamine.
What are the most common side effects of MYQORZO?
The most common side effect of MYQORZO is high blood pressure (hypertension).
These are not all the possible side effects of MYQORZO. Talk to your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Cytokinetics at 1-833-633-2986.
INDICATION AND USAGE
MYQORZO is a prescription medicine used to treat adults with symptomatic obstructive cardiomyopathy (oHCM) to improve functional capacity and symptoms.
It is not known if MYQORZO is safe and effective in children.
Please see full Prescribing Information, including Boxed WARNING, and Medication Guide.



